Ships use ballast water to provide stability and maneuverability during a voyage. Water is taken on at one port when cargo is unloaded and usually discharged at another port when the ship receives cargo. When evaluating ballast water treatment options a number of general factors must be considered including cost, enforcement, the effectiveness of the method, and the risks the treatment may pose to human health and the environment. Current ballast water regulations usually recommend minimizing the risk of introducing non-native species by exchanging ballast water in the open ocean.
Ballast water contains a variety of organisms including bacteria and viruses and the adult and larval stages of the many[ds_preview] marine and coastal plants and animals. While the vast majority of such organisms will not survive to the point when the ballast is discharged, some may survive and thrive in their new environment. These ‘non-native species’, if they become established, can have a serious ecological, economic and public health impact on the receiving environment.
The International Maritime Organization (IMO) has developed international legislation, the International Convention for the Control and Management of Ships’ Ballast Water and Sediments, to regulate discharges of ballast water and reduce the risk of introducing non-native species from ships’ ballast water.
The requirement for ballast water treatment has arisen from the requirements of regulation D-2 of the Convention. In response to this, a number of technologies have been developed and commercialised by different vendors.
Regulation
Ballast water quality and standards
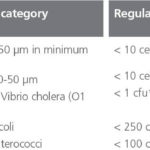
Regulation D-2 of the Ballast Water Convention sets the standard that the ballast water treatment systems must meet (Table 1). Treatment systems must be tested and approved in accordance with the relevant IMO Guidelines.
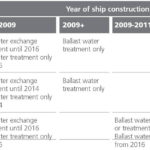
Ships will be required to treat ballast water in accordance with the timetable shown in Table 2. According to this table, the first key milestone was in 2009, when ships under construction during or after that date having less than 5,000 m3 ballast water capacity were required to have ballast water treatment installed to meet the D2 Standard in the Convention. However, as the Convention is not yet in force internationally, these dates cannot be enforced at present.
Ship construction refers to a stage of construction where:
• The keel is laid or construction identifiable with the specific ship begins; or
• Assembly of the ship has commenced comprising at least 50 tonnes or 1% of the estimated mass of all structural material, whichever is less; or
• The ship undergoes a major conversion.
Major conversion means a conversion of a ship:
• which changes its ballast water carrying capacity by 15 percent or greater or which changes the ship type, or
• which, in the opinion of the Administration, is projected to prolong its life by ten years or more, or
• which results in modifications to its ballast water system other than component replacement-in-kind.
Conversion of a ship to meet the provisions in the Convention relating to ballast water exchange (»regulation D-1«) does not constitute a major conversion in relation to the above requirements.
–
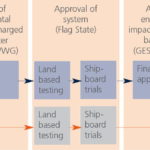
Approval process
The Flag State issuing the approval will mostly choose a classification society to verify and quality assure the tests and resulting data.
Active Substances (AS) are defined by the IMO as »A substance or organism, including a virus or a fungus that has a general or specific action on or against harmful aquatic organisms and pathogens«.
For systems using AS basic approval by the GESAMP (Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection) Ballast Water Working Group (BWWG) is required before shipboard testing. Flag State will issue Type Approval Certificate according to GESAMP approval.
In case of no AS used, Flag State will issue Type Approval Certificate without GESAMP approval.
Treatment Process
Background
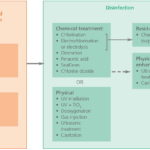
The technologies used for treating ballast water are generally derived from municipal and other industrial applications; however their use is constrained by key factors such as space, cost and efficacy (with respect to the IMO discharged ballast water standards). There are two generic types of process technology used in ballast water treatment: solid-liquid separation and disinfection (Fig. 2).
olid-liquid separation is simply the separation of suspended solid material, including the larger suspended micro-organisms, from the ballast water, either by sedimentation (allowing the solids to settle out by virtue of their own weight), or by surface filtration (removal by straining; i. e. by virtue of the pores in the filtering material being smaller than the size of the particle or organism).
Disinfection removes and/or inactivates micro-organisms using one or more of the following methods:
• chemical inactivation of the microorganism
• physicochemical inactivation by irradiation with ultraviolet light, which denatures the DNA of the micro-organism and therefore prevents it from reproducing. Ultrasound or cavitation (termed »microagitation« for the purposes of this publication) are also physico-chemical disinfection methods
• deoxygenation is achieved by reducing the partial pressure of oxygen in the space above the water with an inert gas injection or by means of a vacuum which asphyxiates the microorganisms.
All of the above disinfection methods have been applied to ballast water treatment, with different products employing different unit processes. Most commercial systems comprise two stages of treatment with a solid-liquid separation stage being followed by disinfection (Fig. 2), though some disinfection technologies are used in isolation. One ballast water treatment technology also employs chemical enhancement (ie coagulation / flocculation) upstream of solid-liquid separation; another uses titanium dioxide (TiO2) to intensify ultraviolet irradiation.
There are two types of ballast water treatment processes, which mostly are combined: solid- liquid separation (filters) and disinfection.
Separation processes
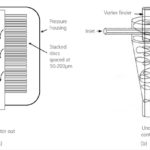
As previously stated, the chemical or physicochemical unit processes used for disinfection are usually preceded by physical solid-liquid separation, by either filtration or hydrocyclone technology. The filtration processes used in ballast water treatment systems are generally of the automatic backwashing type using either discs (Fig. 3a) or fixed screens. Since the standards relating to treated ballast water are size-based, technologies capable of removing materials above a specific size are most appropriate.
Removal of larger organisms such as plankton (Table 1) by filtration requires a filter of equivalent mesh size between 10 and 50 m. Such filters are the most widely used solid-liquid separation process employed in ballast water treatment, and their effective operation relates mainly to the flow capacity attained at a given operating pressure. Maintaining the flow normally requires that the filter is regularly cleaned, and it is the balance between flow, operating pressure and cleaning frequency that determines the efficacy of the filtration process. In principle, surface filtration can remove sub micron (i. e. less than 1 m in size) micro-organisms. However, such processes are not viable for ballast water treatment due to the relatively low permeability of the membrane material.
Hydrocyclone technology is also used as an alternative to filtration, providing enhanced sedimentation by injecting the water at high velocity to impart a rotational motion which creates a centrifugal force (Fig. 3b) which increases the velocity of the particle relative to the water. The effectiveness of the separation depends upon the difference in density of the particle and the surrounding water, the particle size, the speed of rotation and residence time. Since both hydrocylcones and filters are more effective for larger particles, pre-treatment with coagulants to aggregate (or »flocculate«) the particles may be used upstream of these processes to increase their efficacy.
However, because flocculation is time dependent, the required residence time for the process to be effective demands a relatively large tank. The processes can be advanced, however, by dosing with an ancillary powder of high density (such magnetite or sand) along with the coagulant to generate flocs which settle more rapidly.
This is sometimes referred to as »ballasted flocculation«, and is used in some municipal water treatment installations where space is at a premium and has been used in one of the systems included in this publication.
Disinfection
Chemical disinfection
A number of different chemicals or chemical processes have been employed in the ballast water treatment systems reviewed including:
• Chlorination
• Electrochlorination
• Ozonation
• Chlorine dioxide
• Peracetic acid
• Hydrogen peroxide
• Menadione / Vitamin K
The efficacy of these processes varies according to the conditions of the water such as pH, temperature and, most significantly, the type of organism. Chlorine, whilst relatively inexpensive is virtually ineffective against cysts unless concentrations of at least 2 mg/l are used.
Chlorine also leads to undesirable chlorinated byproducts, particularly chlorinated hydrocarbons and trihalomethanes. Ozone yields far fewer harmful byproducts, the most prominent being bromate, but requires relatively complex equipment to both produce and dissolve it into the water. Chlorine dioxide is normally produced in situ, although this presents a hazard since the reagents used are themselves chemically hazardous.
Peracetic acid and hydrogen peroxide (provided as a blend of the two chemicals in the form of the proprietary product Peraclean) are infinitely soluble in water, produce few harmful byproducts and are relatively stable as Peraclean. However this reagent is relatively expensive, is dosed at quite high levels and requires considerable storage facilities.
For all these chemicals pre-treatment of the water with upstream solid-liquid separation is desirable to reduce the »demand« on the chemical, because the chemical can also react with organic and other materials in the ballast water.
Post-treatment to remove any residual chemical disinfectant, specifically chlorine, prior to discharge using a chemical reducing agent (sodium sulphite or bisulphite) may be appropriate if high concentrations of the disinfectant persist. In potable water treatment this technique is routinely employed. When used in ballast water treatment, dosing to around 2 mg/l of chlorine can take place, leaving a chlorine residual in the ballast water tanks to achieve disinfection. The chlorine level is then reduced to zero (»quenching« the chlorine completely) prior to discharge. This technique is used in at least two of the ballast water treatment systems currently reviewed.
Menadione, or Vitamin K, is unusual in that it is a natural product (although produced synthetically for bulk commercial use) and is relatively safe to handle. It is marketed for use in ballast water treatment under the proprietary name Seakleen® by Vitamar, LLC. As with other disinfectant chemicals, it is not without a history of application elsewhere and has been used in catfish farming where it is liberally spread into water. Over three tonnes of menadione are used annually for this application alone.
Physical disinfection
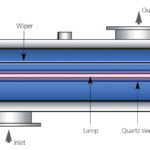
Of the physical disinfection options ultraviolet irradiation (UV) is the most well established and is used extensively in municipal and industrial water treatment applications. The process employs amalgam lamps surrounded by a quartz sleeve (Fig. 3) which can provide UV light at different wavelengths and intensities, depending on the particular application. It is well known to be effective against a wide range of microroganisms, including viruses and cysts, but relies on good UV transmission through the water and hence needs clear water and unfouled clean quartz sleeves to be effective.
The removal of water turbidity (i.e. cloudiness) is therefore essential for effective operation of the system. UV can be enhanced by combining with another reagent, such as ozone, hydrogen peroxide or titanium dioxide which will provide greater oxidative power than either UV or the supplementary chemical reagent alone.
The remaining physical disinfection processes do not inherently require use of pre-treatment. However, the efficacy of both processes is subject to limitations. Deoxygenation takes a number of days to come into effect due to the length of time it takes the organisms to be asphyxiated. However, most voyages will exceed this time period so this should not be a significant constraint.
Cavitation or ultrasonic treatment processes both act at the surface of the micro-organism and disrupt the cell wall through the collapse of microbubbles. Although not used extensively in conventional water / wastewater treatment processes, systems which use these technologies have been awarded Type Approval certificates as of February 2010.
Ballast water treatment unit processes
The range of unit processes employed for ballast water treatment is shown in Table 3. The commercial systems differ mainly in the choice of disinfection technology and the overall system configuration (i. e. the coupling of the disinfection part with solid liquid separation, where the latter is used). Almost all have their basis in land-based systems employed for municipal and industrial water and wastewater and thus can be expected to be effective for the duty of ballast water, albeit subject to constraints in the precise design arising from space and cost limitations.
Treatment technologies and suppliers
Suppliers
This publication considers only suppliers of complete systems for ship based ballast water treatment rather than suppliers of unit operations, although individual proprietary unit operations (e.g. filters, electrochlorination devices, disinfectant chemicals and UV sterilisers) may be included as part of the systems reviewed.
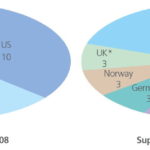
Basic technical information is available from 47 companies, and 40 of these took part in the survey, to produce this February 2010 edition, compared to 28 respondents in September 2008. This is a 42 % increase the 18 months since the guide was last updated.
Information from each of the 40 companies which responded in detail to the survey (Table 3) is presented in the Annex. Where available on web sites, or from other sources, information on the other seven companies listed in Table 3 has been incorporated into the guide. Of the suppliers, around one third are part of a multi-billion dollar turnover international group of companies with significant activity in marine and/or engineering areas. The remainder appear to be SMEs (small to medium enterprises, generally defined as having less than 250 employees) all of which have been set up within the past 15 years. Fourteen different countries are represented by these 40 companies, with the predominant nation being the US (Fig. 5).
It is apparent from Fig. 5 that since September 2008, the number of suppliers of ballast water treatment systems and the number of countries in which they are based has increased significantly.
Technologies
The combination of treatment technologies utilised by the various suppliers are summarised in Table 3; since one supplier Hyundai offers two systems, there are 41 systems in total. All of the products for which information is available, other than those based on gas injection, are either modular or can be made so.
All of the systems reviewed have undergone preliminary pilot trials. The published data from these trials has shown the systems to be generally effective with reference to the IMO treated water standards applicable to discharged ballast water shown in Table 1.
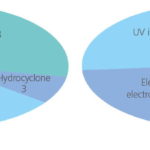
Of the systems considered the majority employ upstream filtration for solid-liquid separation (Fig. 6a), with the filter pore size primarily in 30–50 m range. Only one system (Hitachi) employs pre-coagulation upstream of the filter. This particular system employs magnetic particles to accelerate the clarification process (»enhanced flocculation«). A magnetic separator is then used prior to filtration to remove particles. One supplier uses cartridge filters which are not backwashable. Three suppliers employ hydrocyclones.
All solid-liquid separation processes produce a waste stream containing the suspended solids. This waste stream comprises the backwash water from filtering operations or the underflow from the hydrocyclone separation. These waste streams require appropriate management. During ballasting they can be safely discharged at the point where they were taken up.
On deballasting, the solid-liquid separation operation is generally by-passed.
Whilst there are a range of disinfection processes used for ballast water treatment, the majority of the systems are based on either electrolytic treatment (electrolysis or electrochlorination) or UV irradiation (Fig. 6b). In one case (Alfa Laval system), the UV irradiation is supplemented with titanium dioxide (TiO2) to intensify the oxidative power of the UV light.
The electrolytic treatment products have different design features but all essentially employed a direct current to electrolyse the water. Electrolytic technologies provided for ballast water treatment may be designed to generate either chlorine, as in the classic electrochlorination process, or other oxidative products. Those designed for chlorine generation rely on the salinity of the feedwater for effective chlorine generation; supplementary brine is necessary when the abstracted ballast water is fresh.
This is not an issue for chlorination, of which there are three examples, using either chlorine gas or hypochlorite. There are only single examples of the use of chemicals such as SeaKleen, vitamin K and nonoxidising biocides. One supplier, Sea Knight, uses bioremediation following deoxygenation.
Almost half of the systems reviewed treat the ballast water both during ballasting and discharge (Table 5). If filtration is used with backwashable filters then the filters are by-passed during discharge to avoid discharging non native organisms and other material into the receiving water. The majority of the other technologies treat only during ballasting. Of the remainder, two treat during discharge and others during ballasting and during the voyage.
Cost and footprint
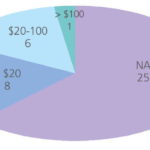
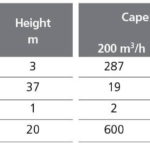
The key technical features of the system with respect to ballast water treatment are the flow capacity, footprint, overall size of the system and costs, the latter comprising capital expenditure (capex) and operating expenditure (opex). Most of the technologies have been developed for a flow rate of about 250 m3/hr, considered to be the flow rate required for the first phase of ships required to be equipped with ballast water treatment technology. Since the systems are largely modular in design (other than the gas injection type), there is no technical limit to the upper flow rate other than that imposed by size and/or cost. In some cases there are examples of systems already installed for flows above 5,000 m3/hr. The mean key data for costs and footprint for all the technologies are summarised in Table 4 and Figures 7 and 8. Full data are provided in Table 5. The mean quoted estimated or projected operating cost of the systems, on the basis of the 16 sets of data provided is $ 30 per 1,000 m3, within a broad range of values from no cost (when waste heat is used) to $ 130 per 1,000 m3 treated water. Eight of the 16 suppliers who provided operational expenditure information quoted costs below $ 20 per 1,000 m3, and variation may be due to methods of calculating opex. Some suppliers indicated that extra water head on ballast pumps may be required. There is a tendency, where data is available, for larger units to be more efficient in terms of power requirements, which for the 33 systems for which data was available ranged from 0 to 220 kW per 1,000 m3 of treated ballast water.
In most cases (except for the few technologies that use stored chemicals and the gas injection units that use fossil fuel) the majority of the opex relates to the power required to operate the process (UV irradiation, electrolysis or ozonation).
Capital cost information is more widely available in 2010 compared to 2008, however, just over half of the suppliers regard this information as confidential. From the 19 sets of data provided, the capital cost of a 200 m3/h plant ranges from $ 2,000 (by Mexel Industries) to $ 600,000, with a mean value of around $ 20,000, which is $ 100,000 less than in September 2008. For a 2,000 m3/h plant, the equivalent values are $ 5,000 (again Mexel) to $ 2,000,000 with a mean of $ 780,000, also lower than 2008. As with the opex, from the limited information provided there appears to be no correlation between the quoted capex and the configuration of the process, and variations in price arise from differences in assumptions made by the various suppliers regarding inclusion or exclusion of specific components. Prices quoted must be regarded as tentative since some of these products are still under development and the price is to some extent determined by the marketplace.
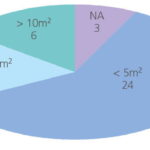
The footprint of the systems reviewed varies between 0.25 and 30 m2 for a 200 m3/h unit, with a mean value of 7 m2, according to the data provided by suppliers in relation to 37 systems. For a unit of ten times this flow capacity, there is less information, since some suppliers do not provide units of this size, and the minimum, maximum and mean values are 1, 145 and 21 m2 respectively. One supplier (Atlas) gave data for both the control panel / electrolysis system and the pre-filter. Optimarin stated that their system may be suspended under the deck, giving a zero footprint. Thus, whilst the units may be predominantly modular, this does not imply that the footprint increases proportionately with flow capacity.
Other system characteristics
Other technical features of the products are not necessarily common to all of them and are specific to generic types of process technology. These process-specific facets can be summarised as follows:
• Deoxygenation is the only technology specifically developed for ballast water treatment and is effective because the de-aerated water is stored in sealed ballast tanks. However the process takes between one and four days to take effect, and thus represents the only type of technology where voyage length is a factor in process efficacy. This type of technology is also the only one where, technically, a decrease in corrosion propensity would be expected (and, according to one supplier, has been recorded as being suppressed by 50–85 %), since oxygen is a key component in the corrosion process. The water is re-aerated on discharge.
• Systems in which chemicals are added normally need to be neutralised prior to discharge to avoid environmental damage in the area of discharge. Most ozone and chlorine systems are neutralised but some are not. Chlorine dioxide has a half life in the region of 6–12 hours, according to the supplier, but at the concentrations at which it is employed it can be safely discharged after a maximum of 24 hours.
• Essentially most UV systems operate using the same type of medium pressure amalgam lamps. A critical aspect of UV effectiveness is the applied UV dose/power of the lamp. This information has not been given by all suppliers. Another aspect of UV effectiveness is the clarity of the water. In waters with a high turbidity or colloidal content, UV would not be expected to be as effective.
• Most chlorination systems are applying a dose in the region of 2 mg/l residual chlorine which has proven to be effective.
• Most ozonation suppliers are using an ozone dose of 1–2 mg/l which has proven to be effective.
• UV systems are the least complex treatment plants to operate. Electrolysis and electrochlorination plants are the most complex.
• Deoxygenation plants are relatively simple devices if an inert gas generator is already installed on the ship and in the latter case would take up little additional space.
• The biggest operating cost for most systems is power and for large power consumers (electrolytic and advanced oxidation processes) availability of shipboard power will be a factor.
• For chemical dosing systems, power is very low and chemical costs are the major factor. For these reasons chemical addition may be better suited to small ballast capacities.
• Although the systems operate at generally low pressure and thus do not require additional ballast water pumping pressure, those employing venture devices (for exerting shear) incur pressure losses of up to 2 bar.
• For most systems it is recommended that installation takes place in the engine/machine room near the existing ballast water pumps, although installation on deck may also be possible if appropriate precautions are taken. If the location is in an explosion zone, then the installation will need explosion proofing and one supplier, Techcross, has Type approval for an explosion proof system. The generation of hydrogen by the electrolytic technologies is not considered an issue, since the gas is vented and diluted with air to safe levels.
• Whilst disinfection by-products are an issue, and central to the approval of ballast water management systems that make use of active substances, suppliers are confident that the levels generated are unlikely to be problematic. There is a large amount of scientific and technical information on disinfection by-products formation that is likely to support this.
Commercial availability
By February 2010, 27 suppliers stated that they had systems installed on ships. A total of 106 ballast water treatment systems had been installed by these suppliers as of February 2010, an increase of 50 systems over the 18 months since the last update to this guide. UV based systems, from Hyde Marine and Optimarin account for around 25 % of installations, and electrochemical systems, from RWO Marine and Techcross accounting for a further 25 %.
Approval status
The regulatory framework requires that a key distinction is to be made between those systems employing active substances (primarily disinfectant chemicals) and those which do not. Non-AS systems would appear to have less regulatory hurdles to overcome as they do not require GESAMP G9 approval. However, a number of manufacturers have successfully demonstrated that it is possible to obtain full type approval certification ahead of systems which do not require GESAMP approval, as five of the eight systems with type approval certificates have been through the G9 approval process.
According to information provided by the suppliers, an increasing number of the technologies reviewed are progressing towards approval, though the scheduling of the testing differs between the different suppliers and thus the projected date for final approval. To date 18 of the active substance systems have received basic approval from the MEPC, however, a further four are expecting basic approval at MEPC 60. As of February 2010, 10 of these have obtained final approval, with a further three expected to gain this at MEPC 60. Further approvals are likely at subsequent MEPC meetings, with manufacturers projecting approvals in both 2010 and 2011. It is clear, however, that many systems are undergoing »G8« ballast water management systems approval without having received basic approval for the active substances. Indications are that up to twelve companies are or will be undertaking testing of ballast water management systems at test facilities during 2010 and 2011.
By February 2010, eight systems had received type approval certificates, one of which (Techcross) also has type approval for an explosion proof system. Suppliers of three systems state that they expect type approval in early 2010 and a further nine project dates between late 2010 and 2012.
Concluding remarks
The previous edition of this ballast water treatment technology guide predicted that the number of systems with type approval would »significantly increase over the next 12–18 months«. In fact, since September 2008, the number of such systems has almost trebled, from three to eight.
The systems that have obtained type approval demonstrate that a wide range of technologies, with or without the use of active substances, are suitable for the treatment of ballast water to the standards required by the G8 guidelines. The use of active substances and the need to undergo the approval process specified in the G9 guidelines do not present a significant barrier to obtaining type approval.
It is now apparent that technologies to treat ballast water to meet the D2 standard within the International Convention for the Control and Management of Ship’s Ballast Water and Sediments are available and established, with over one hundred such systems installed worldwide.
Peter Catchpole